The Centers for Medicare and Medicaid Services (CMS) has released its 2025 Medicare Advantage and Part D Final Rule, a massive document totaling 1,327 pages (https://public-inspection.federalregister.gov/2024-07105.pdf). This ruling introduces new marketing regulations for Third Party Marketing Organizations (TPMOs), focusing on the requirement for prior express written consent on a very strict basis. The CMS’s enforcement date is set for October 1, 2024, aligning with the start of the annual Medicare open enrollment period, even though the official rules take effect in January 2025. There is nothing like enforcing major new rule changes, with limited time to comply, on an industry that moves like an aircraft carrier making a sharp turn.
What could possibly go wrong?
Retroactive Nature of the Rule
The CMS’s enforcement date of October 1, 2024, applies to TPMOs even if they collect data before this date. This retroactive approach emphasizes the need for clear, written consent for each instance of data sharing, regardless of when the record was created. This aspect is significant because it closes potential loopholes where previously gathered data could be used without updated consent. Have you been applying the new 1:1 requirement in your marketing to date? If not, those leads and records are potentially cooked.
Why the Go-Live Date Is So Critical
The October 1 enforcement date is crucial because it aligns with the start of the annual Medicare open enrollment period. This is when TPMOs often engage in large-scale marketing to attract new enrollees or retain existing ones. The retroactive requirement for written consent aims to ensure these marketing activities are significantly impacted—there’s no other reason for the retroactive application.
Removal of Manual Dialing Exemption
The CMS’s new rules remove the exemption for manual dialing, meaning TPMOs must now obtain prior express written consent even when dialing manually. This change reinforces the one-to-one consent structure and ensures beneficiaries are protected from unwanted marketing practices, whether automated or manually dialed. It’s another huge blow.
The One-to-One Consent Structure
The CMS’s ruling requires TPMOs to follow a “one-to-one” consent structure, meaning personal beneficiary data cannot be shared with another TPMO unless prior express written consent is obtained on a one-to-one basis, which is a big shift in how consent has been collected to date. This in and of itself has a wide ranging impact on everyone involved. This aligns with the FCC’s Second Report and Order, specifying that consent must be clear, in writing, and authorize only one identified seller. This approach aims to reduce unwanted marketing communications and increase accountability among TPMOs. What it will really do is create a seismic shift in the lead generation, digital marketing, and advertising spaces.
Enforcement of the FCC Ruling
The CMS’s ruling enforces the FCC’s significant TCPA changes aimed at closing the lead generator loophole in the marketing space. This enforcement is notable because it applies even though the rule isn’t officially active until January 2025. To understand the full scope of the FCC’s efforts to address aggressive marketing, check out our analysis: “The FCC’s New Crackdown: A Tidal Wave of Challenges for Businesses and Lead Generation.”
Implications for Carriers and TPMOs
These new rules have significant implications for Third Party Marketing Organizations (TPMOs) and Carriers (payors). With the retroactive enforcement of prior express written consent applied to all calls, including manually dialing, both TPMOs and Carriers must adapt to comply with these regulations.
Here’s how these changes impact TPMOs and Carriers:
- For TPMOs: They must now obtain prior express written consent even when manually dialing, which was previously exempt. This compliance challenge will dramatically affect their marketing strategies, requiring adjustments to avoid penalties and reputational damage. The CMS’s focus on compliance during open enrollment underscores the importance of meeting these new standards. The aggressive retroactive application of data requirements adds significant challenges and risks here.
- For Carriers (Payors): The new rules place more liability on Carriers for the actions of their downstream partners, TPMOs. This means Carriers must ensure their partners comply with the CMS’s requirements, similar to how telco Carriers are held responsible for policing their own downstream operators. This could lead to Carriers taking a more conservative approach with TPMOs, impacting their ability to market effectively. In reality, this might put many TPMOs out of business altogether.
Special Thanks
A special thanks to Troutman and Amin, who have been instrumental in analyzing and providing insights into these regulatory changes. They hosted a webinar on April 24, 2024, detailing the implications of the new CMS rules and offering guidance for compliance. Their expertise has been invaluable in helping the industry navigate these complex changes.
Final Thoughts
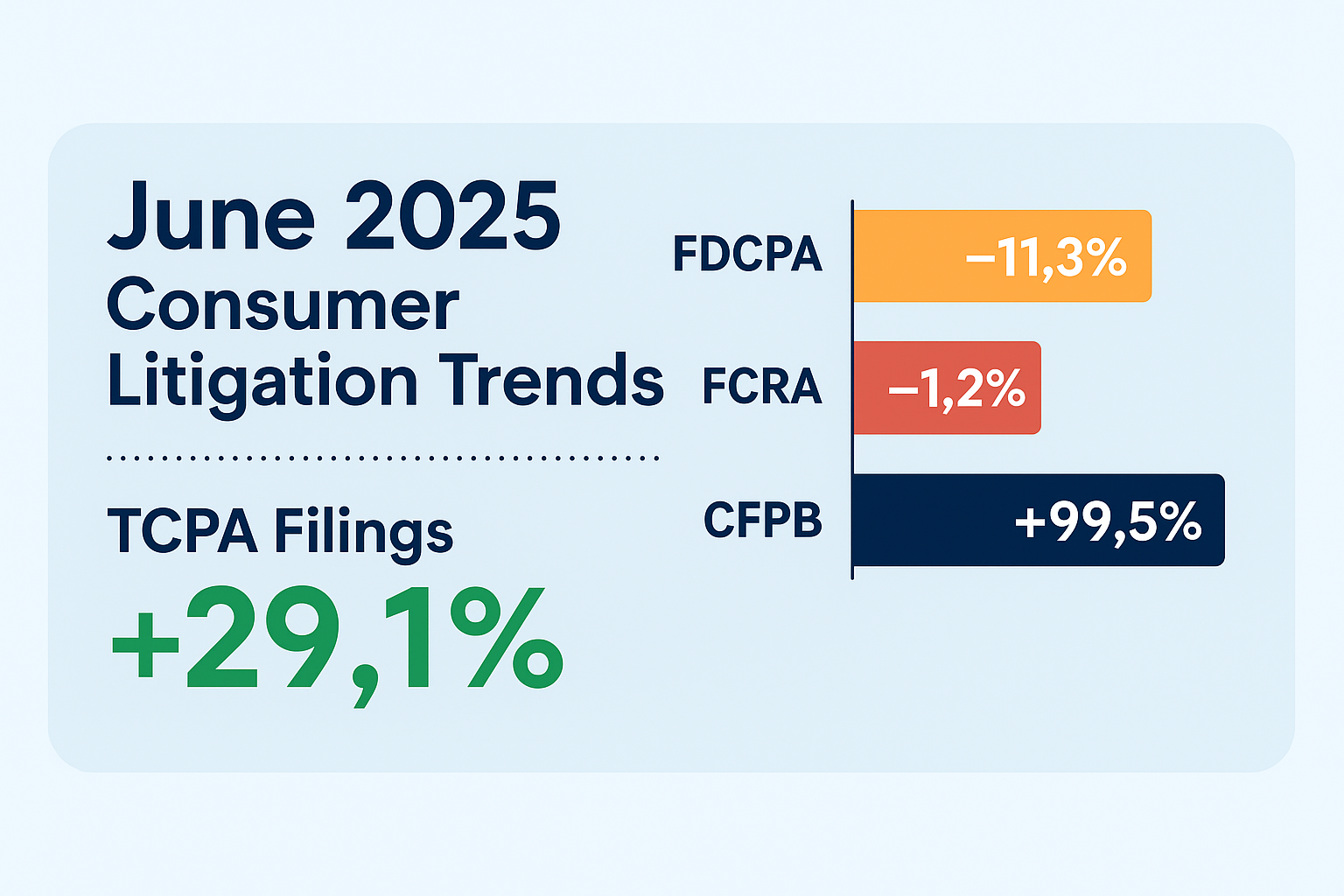
The CMS’s Final Rule introduces significant challenges for TPMOs, with a focus on consumer protection and clear consent. The retroactive requirement for prior express written consent, especially as open enrollment is set to kick off, signals a move towards greater accountability and transparency. This could open the door to a massive increase in TCPA suits, which have already been growing year after year. Applying the same pressure to the payors as the FCC has applied to the telco carriers, forcing them to police their own downstream operators, is a big shift with major implications. As the healthcare industry, especially TPMOs, adjusts to these new rules, the industry as a whole could see some significant shifts — whether they turn out good or bad (and for whom), only time will tell. These kinds of sweeping changes, with extremely limited time to adjust, always result in unintended consequences. Compliance and liability are guaranteed to be one of the biggest outcomes, that is for sure.